Immune atomic force microscopy of the inner ear motor protein prestin using Qdots
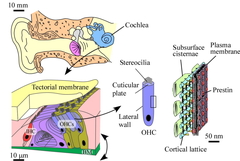
Fig. 1. Human auditory system. Sensory cells, such as outer hair cells (OHCs), inner hair cells and various kinds of the other cells, sit on the basilar membrane (BM). OHCs subject the membrane to force, leading to cochlear amplification, resulting in the high sensitivity of mammalian hearing. Prestin is thought to be the origin of the motility of OHCs.
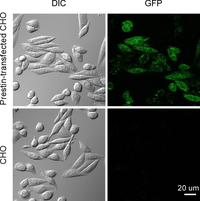
Fig. 2. GFP fluorescence of prestin-transfected and untransfected CHO cells. Left panels, differential interference contrast (DIC) images. Right panels, GFP fluorescence images. GFP fluorescence was detected in the prestin-transfected cells (top right), indicating the expression of prestin in the cells.
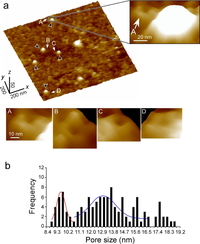
Fig. 3. Membrane topology of prestin on the cytoplasmic face of the plasma membrane of the prestin-transfected CHO cells. (a) High-magnification 3-D AFM images. Similar structures were observed at the locations indicated by arrows (A–D). (b) Frequency distribution of the diameter of the ring-like structures (n = 120) found around the Qdots (n = 109) on the isolated plasma membranes of the prestin-transfected CHO cells (n = 23). This histogram exhibited two main Gaussian distributions shown by red and blue lines. The peaks of those distributions were 9.6 ± 0.1 nm (red) and 13.0 ± 0.2 nm (blue), respectively.
Prestin, a membrane protein of the outer hair cells (OHCs), is known to be the motor which drives OHC somatic electromotility (Fig.1). Morphologically, the lateral walls of the OHCs have been shown by electron microscopy to be densely covered with particles about 10 nm in diameter (Forge, Cell Tissue Res. 265:473-483, 1991), these particles being believed to be the motor protein prestin. Imaging by atomic force microscopy (AFM) of prestin-transfected Chinese hamster ovary (CHO) cells has revealed particle-like structures 8-12 nm in diameter to possibly be prestin (Murakoshi et al., J. Assoc. Res. Otolaryngol. 7:267-278, 2006). However, since there are many kinds of intrinsic membrane proteins other than prestin in the plasma membranes of OHCs and CHO cells, it was impossible to clarify which structures observed in such membranes were prestin.
In the present study, an experimental approach combining AFM with quantum dots (Qdots), termed "immune atomic force microscopy," was newly developed to detect individual prestin molecules.
Prestin-transfected CHO cells (Iida et al., JSME Int. J. 46C:1266-1274, 2003) were used (Fig. 2). Inside-out plasma membranes were isolated from the prestin-transfected and untransfected CHO cells. Such membranes were then incubated with anti-prestin primary antibodies and Qdot-conjugated secondary antibodies. The plasma membranes of both types of CHO cells were subsequently scanned by AFM.
Figure 3a shows high-magnification 3-D AFM images of a prestin-transfected CHO cell. Qdots were not seen in the AFM images of the untransfected CHO cells. However, Qdots, about 8 nm in height, were clearly seen in the prestin-transfected CHO cells (black arrows). Squarish ring-like structures, each with four peaks and one valley at its center, were observed in the vicinity of the Qdots (right), suggesting that prestin forms a tetramer in the plasma membranes of the prestin-transfected CHO cells. Analysis of the size of the observed structures showed two different sizes of prestin, i.e., 9.6 and 13.0 nm (Fig. 3b), suggesting the existence of the compact- and extended-state prestin.
